batteries
The most common types today include lithium-ion batteries (known for their high energy density and long lifespan, they are widely used in portable electronics and EVs), lead-acid batteries (one of the oldest types of rechargeable batteries, used in vehicles and for backup power systems due to their reliability and cost-effectiveness, and flow batteries (suited for stationary energy storage, such as grid support, because they can scale easily and offer long discharge times). Advancements in battery technology focus on increasing energy density, reducing costs, and utilizing materials that are abundant and environmentally friendly.

Acid scavengers play a crucial role in enhancing the performance and safety of lithium-ion batteries, particularly in the context of the electrolyte solution used in these batteries. By incorporating acid scavengers into the electrolyte of lithium-ion batteries helps maintain the integrity of the battery’s internal architecture, ensuring that the electrodes and separator remain intact and functional throughout the battery's operational life. This stabilization of the internal environment not only improves the battery's performance—by maintaining optimal ionic conductivity and reducing resistance—but also significantly enhances its safety. By reducing the formation of hydrofluoric acid and other corrosive substances, acid scavengers decrease the risk of internal chemical degradation, which is a common cause of battery failures and safety incidents, such as fires and explosions.
ion exchange resin, acid scavenger
Acid scavengers are integral to the longevity, performance, and safety of lithium-ion batteries. They effectively neutralize harmful acidic impurities within the electrolyte, protecting the battery’s critical internal components from chemical damage. As lithium-ion technology continues to advance, enhancing the functionality and compatibility of acid scavengers remains a key area of research and development, aiming to further improve battery efficiency and safety.
Reillex™ Resins are weak-base ion exchange (IX) resins for applications requiring high temperature, oxidative, and strong acid stability, broad metal chelation properties, and excellent acid scavenging capabilities.
Binding agents play a crucial role in the performance and longevity of batteries, especially in lithium-ion and other advanced battery technologies. They are indispensable in the construction and operation of modern batteries. They ensure the mechanical integrity, electrical conductivity, and chemical stability of the electrodes, directly influencing the battery's efficiency, capacity, durability, and overall performance. Binding agents are essential in maintaining the structural integrity of the electrodes within a battery.
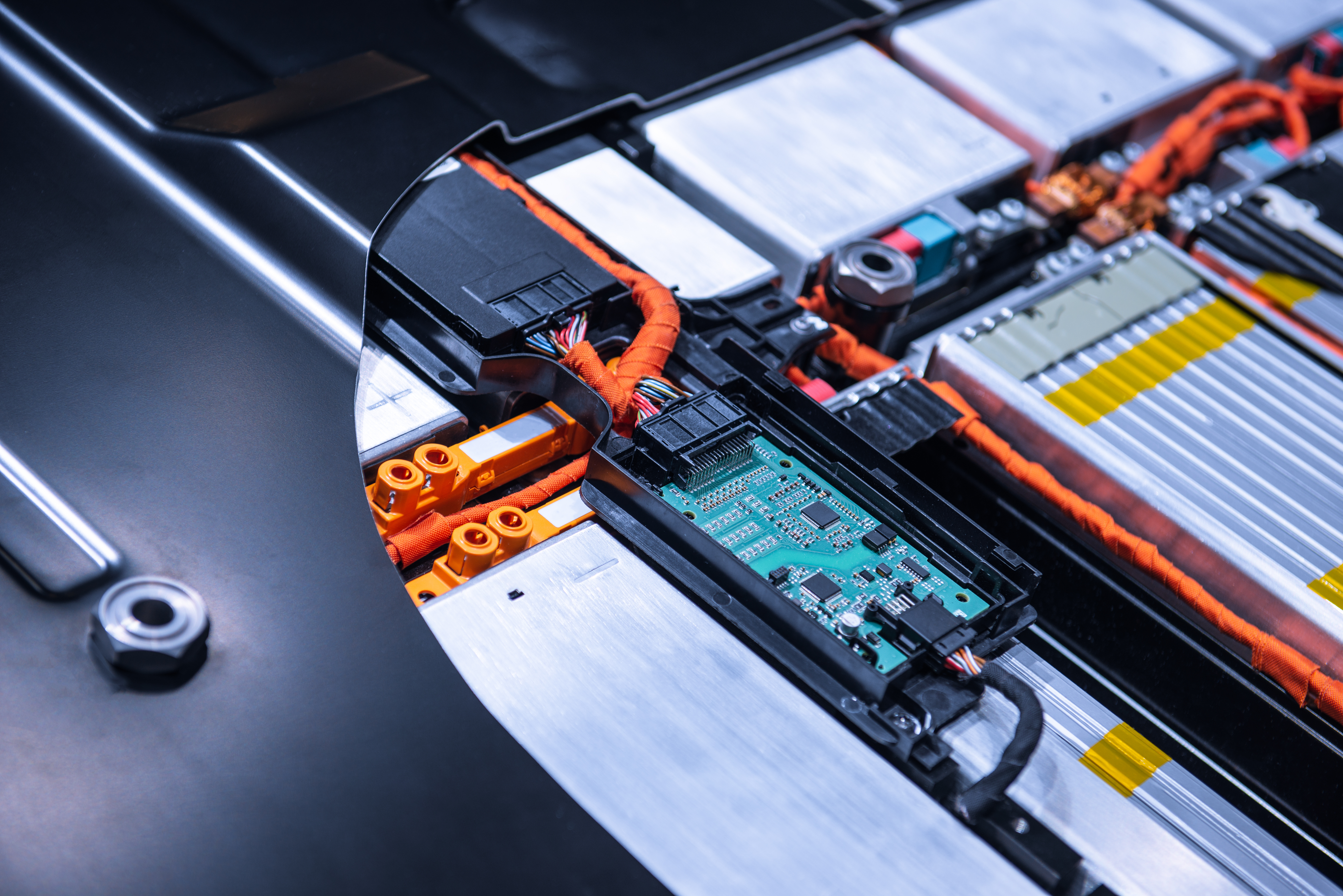
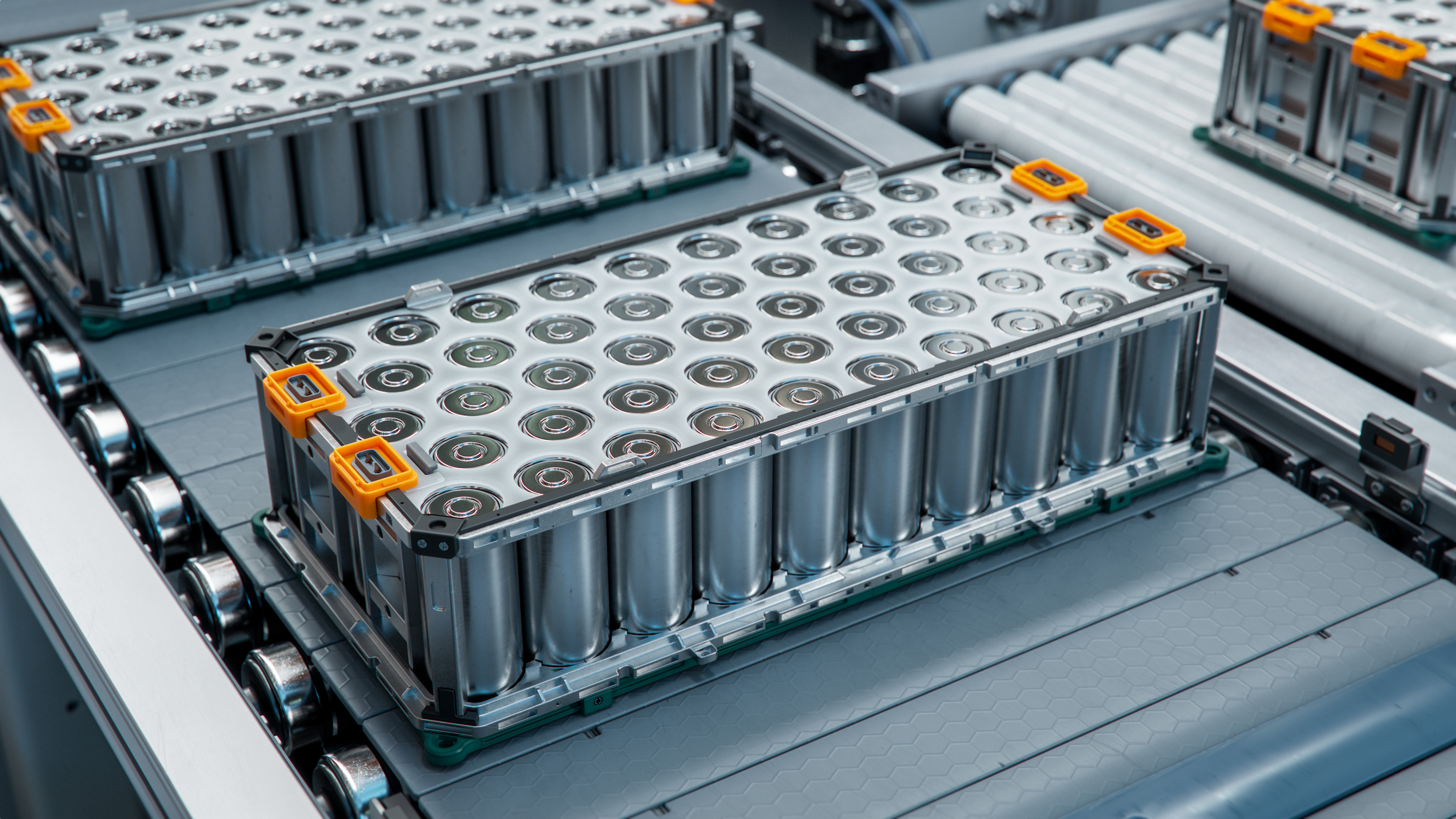
Electrolyte modifiers are critical components in enhancing the performance and safety of batteries, particularly in lithium-ion technologies. These additives are used to modify the electrolyte—the medium through which ions move between the cathode and anode in a battery. As the demand for more efficient and safer batteries continues to grow, particularly in the electric vehicle and renewable energy sectors, the development of innovative electrolyte modifiers remains a key focus in battery research and development.
maleic anhydride, binding agent and electrolyte modifier
In the context of battery manufacturing, especially lithium-ion batteries, Zemac™ Compatibilizers can be used as binding agents and electrolyte modifiers:
- binding agents: They help in holding together the active material and the conductor on the battery's electrodes. This binding is crucial for maintaining the structural integrity of the electrodes and ensuring consistent electrical conductivity throughout the battery's lifecycle.
- electrolyte modifiers: EMA copolymers can be used to modify the electrolyte solutions within batteries. They improve the ionic conductivity of the electrolyte, enhance the battery's overall stability, and reduce the formation of undesirable byproducts during charging and discharging cycles, which can degrade battery performance.
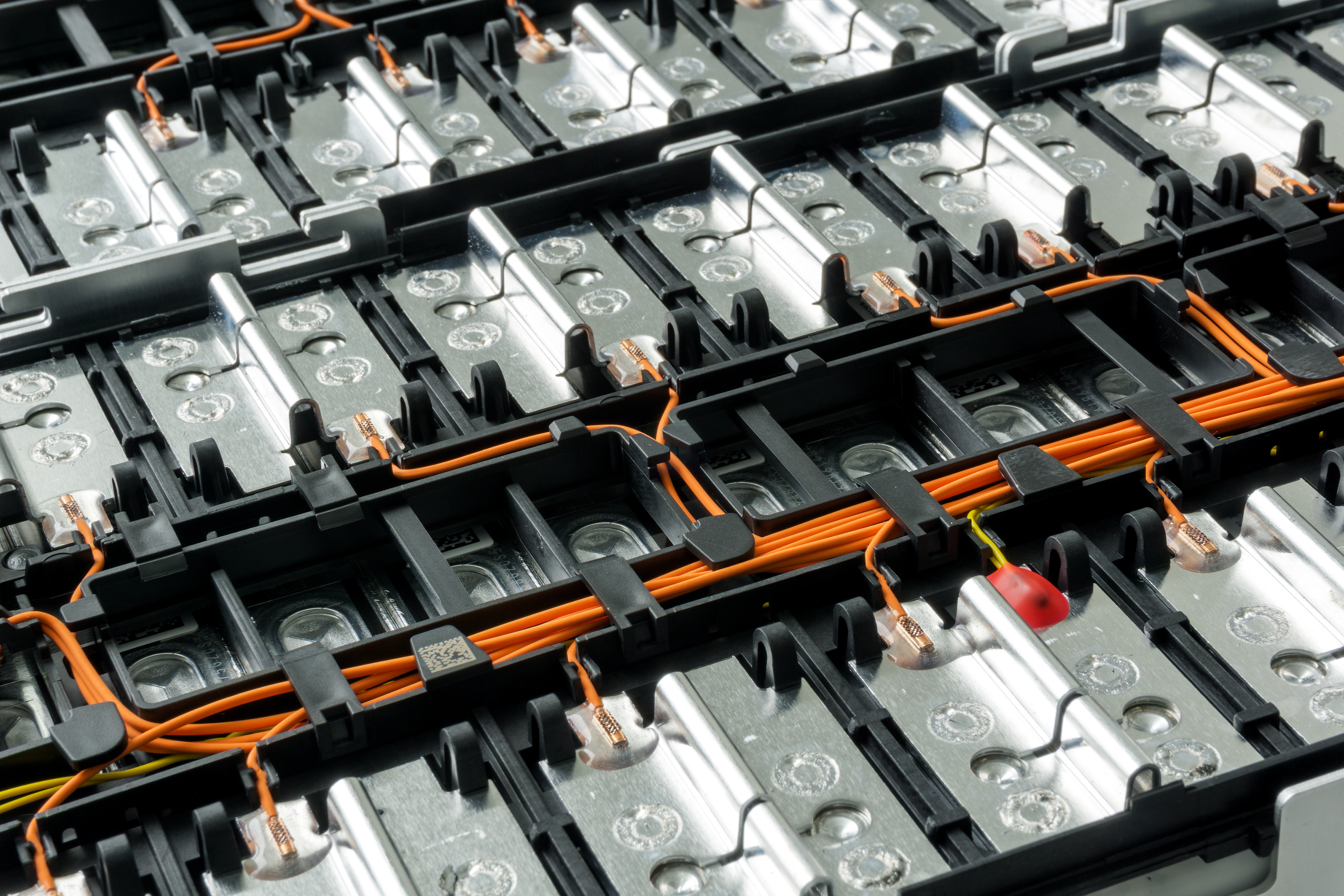
Dispersion agents are essential in battery manufacturing, particularly for ensuring uniform particle distribution and enhancing electrode performance in lithium-ion batteries. They facilitate the mixing and stability of electrode material slurries, preventing sedimentation and ensuring homogeneous coatings on current collectors. This uniformity is crucial for maximizing electrochemical reaction areas, improving adhesion, and reducing internal resistance, which collectively enhances the battery's capacity, efficiency, and longevity. Dispersion agents help lower slurry viscosity for smoother application, prevent particle agglomeration, and support better ion transport, contributing to higher charge and discharge efficiencies and improved overall battery performance.
Dispersion agents are fundamental in the battery manufacturing process, aiding in material processing, enhancing performance characteristics, and ensuring the durability and efficiency of the final product.
styrene maleic anhydride, dispersion agent
SMA™ Copolymers (Styrene Maleic Anhydride) are particularly useful as dispersion agents in battery applications due to their unique chemical properties and functionality. Reasons that SMA Copolymers are effective as dispersion agents in battery manufacturing include:
- hydrophilic-lipophilic balance: SMA Copolymers offer a beneficial balance between hydrophilic (water-attracting) and lipophilic (oil-attracting) properties. This balance is crucial for stabilizing dispersions of hydrophobic particles in aqueous mediums, which is a common requirement in electrode slurry formulations.
- chemical stability: SMA Copolymers are chemically stable under the conditions used in battery manufacturing. They can withstand the range of pH, temperature, and solvent conditions typically encountered, ensuring that they maintain their performance characteristics throughout the battery's manufacturing and operational lifecycle.
- ionic conductivity: When modified, SMA Copolymers can enhance ionic conductivity by facilitating ion transport within the electrode matrix. This is particularly valuable in battery applications where efficient ion transport is critical for high performance.
- compatibility with electrode materials: SMA has good compatibility with various electrode materials used in batteries, including lithium compounds and various conductive carbons. This compatibility helps in forming a uniform and stable coating of the active material on the current collectors.
- adjustable properties: The properties of SMA Copolymers can be adjusted by varying the ratio of styrene to maleic anhydride in the copolymer. This allows for precise control over the copolymer's solubility, glass transition temperature, and other physical properties to tailor it for specific needs in battery manufacturing.
- enhanced adhesion and mechanical strength: SMA enhances the adhesion between the active materials and the binder, as well as the adhesion between the electrode layer and the current collector. Improved adhesion contributes to the mechanical strength and durability of the electrodes, which is important for maintaining performance during repeated charging and discharging cycles.
